How to Calculate Partial Pressure
11 342 kilograms kg of Lead fit into 1 cubic meter. At 25C 77F or 29815K at standard atmospheric pressure.

Partial Pressures Of Gases And Mole Fractions Chemistry Tutorial Youtube
When dealing with gases dissolved in liquids like oxygen in blood partial pressure is the pressure that the dissolved gas would have if the blood were allowed to equilibrate with a volume of gas in a container.

. Before you move further you need to understand important gas laws which are explained below. To use the formula for a real gas it must be at low pressure and low temperature. The ideal gas law is written for ideal or perfect gases.
Vapour pressure is a measure of the ability of a compound to bond with itself. An online partial pressure calculator is properly designed to calculate partial pressure volume temperature and amount of moles of each individual gas enclosed in a container. Scale_height temperature_bottom temperature_top Calculate the scale height of a layer.
The pressure of any gas within the container is called its partial pressure. P tot P i P 1 P 2 P 3. To calculate partial pressure start by applying the equation k PV to treat the gas as an ideal gas according to Boyles law.
DP dT H T ΔV where. Gas mixtures and partial pressures. The partial pressure is defined as the pressure of a single gas component in a mixture of gases.
Calculate the pressure at a certain height above another pressure level. You can use values for real gases so long as they act like ideal gases. Lead weighs 11342 gram per cubic centimeter or 11 342 kilogram per cubic meter ie.
Epaminondas Voutsas in Thermodynamics Solubility and Environmental Issues 2007. Then convert the equation into Kelvin if it isnt already by adding 273 to the temperature in Celsius. P i the.
Partial pressure refers to the pressure exerted on the container walls by a specific gas in a mixture of other gases. It corresponds to the total pressure which the single gas component would exert if it alone occupied the whole volume. Visit BYJUS for more content.
Compound molecules that bond well. Density of water is equal to 1 000 kgm³. Vapor pressure or vapour pressure in English-speaking countries other than the US.
Increasing pressure or temperature raises the kinetic energy of the gas and forces the molecules to interact. The Clapeyron equation states. To picture this speed calculate the.
G G M R 2. H is the specific latent heat - the thermal energy absorbed or released during a phase transition. The partial pressure is defined as the pressure of a single gas component in a mixture of gases.
To Learn expressions on Daltons law of partial pressure Examples Videos with FAQs. The total pressure of the gas mixture is the sum of the partial pressure of the component gases. Definition of partial pressure and using Daltons law of partial pressures.
Relative Density - Relative density is density of an object in terms of the density of another reference object. DPdT is a derivative of pressure with respect to temperature. When the valves are opened the fluid can flow freely resulting in no pressure drop.
See spelling differences or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases solid or liquid at a given temperature in a closed systemThe equilibrium vapor pressure is an indication of a liquids evaporation rate. The following tools allow you to calculate the density of moist air by knowing either the temperature pressure and relative humidity OR temperature dew point and pressure. Vapour pressure of a pure compound is the pressure characteristic at any given temperature of a vapour in equilibrium with its liquid or solid form.
70805793 pounds lbs of Lead fit into 1 cubic foot. This is the. If the answer comes out smaller than the moles of liquid put in the.
The gases present in the container are chemically inert. Density - Measured in Kilogram per Meter³ - The Density of a material shows the denseness of that material in a specific given area. If the valves are closed or any one valve is closed it will change the direction of the fluid flow inside the pipe and will lead to pressure loss.
The cubic feet per minute CFM of a gas describes its volumetric flow rate through a pipe or vent. In Imperial or US customary measurement system the density is equal to 624 pound per cubic foot lbft³ or 058 ounce per cubic inch ozinch³. So what one does is calculate the partial pressure at the given temperature convert using the ideal gas law pVnRT where p is pressure V container volume n moles of vapor R the gas constant T the absolute temperature to figure out how many moles of vapor that would be.
This is taken as mass per unit volume of a given object. At 20C 68F or 29315K at standard atmospheric pressureIn Imperial or US customary measurement system the density is equal. Water Density - Measured in Kilogram per Meter³ - Water Density is mass per.
The theory of the o2 sensor working principle is detailed here. T is the temperature. Density of lead is equal to 11 342 kgm³.
After youve done that begin finding the partial pressure of each gas by using the formula P nRTV. Daltons law of partial pressure. ΔV is the change of the specific volume during a phase transition.
If youre seeing this message it means were having trouble loading external resources on our website. Calculate the saturation water vapor partial pressure. The volumetric flow is a good measure of how much gas goes through the system but it isnt the clearest way of picturing how quickly it moves.
If the valves of the pipe are open there will be no resistance and hence pressure loss will not take place. Gravity R - radius of the planet or object on which you calculate surf. P tot the total pressure.
It corresponds to the total pressure which the single gas component would exert if it alone occupied the whole volume. Partial Pressure- Partial Pressure is defined as a container filled with more than one gas each gas exerts pressure. The theory of the o2 sensor working principle is detailed here.
Add_pressure_to_height height pressure. Using the ideal gas law to calculate a change in volume. The density of moist air is calculated as the sum of the density of the dry air component of the mixture plus the density of the saturated component of the mixture.
Water weighs 1 gram per cubic centimeter or 1 000 kilogram per cubic meter ie. The term partial pressure is used when we have a mixture of two or several gases in the same volume and it expresses the pressure that is caused by each of the induvidual gases in the mixture. How to Calculate CFM to MPH.
This formula is illustrated in a phase diagram - a. Where G - Gravitational constant 66710-11 Newton-meter 2 kg 2 M - mass of the planet or object on which you calculate surf.

Gas Mixtures And Partial Pressures Video Khan Academy

How To Calculate Partial Pressure 14 Steps With Pictures
Mixtures Of Gases Partial Pressures
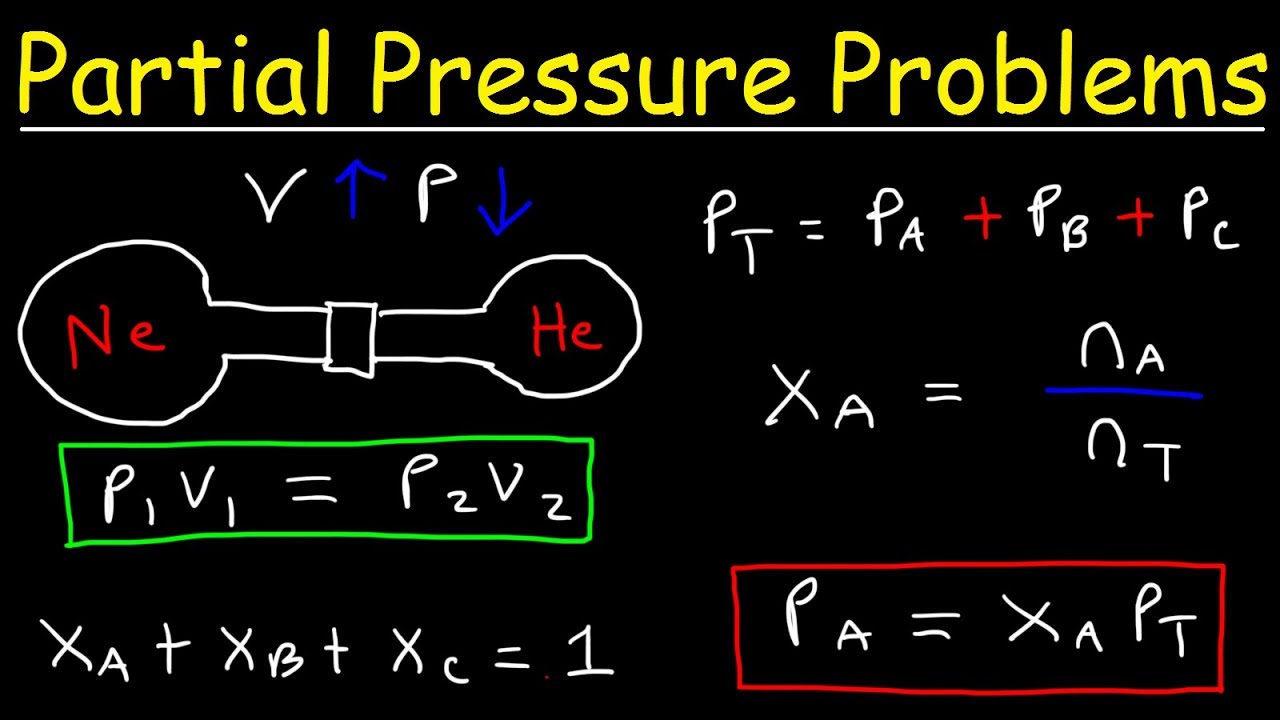
Dalton S Law Of Partial Pressure Problems Mole Fraction Chemistry Gas Laws Youtube
No comments for "How to Calculate Partial Pressure"
Post a Comment